Market Insights
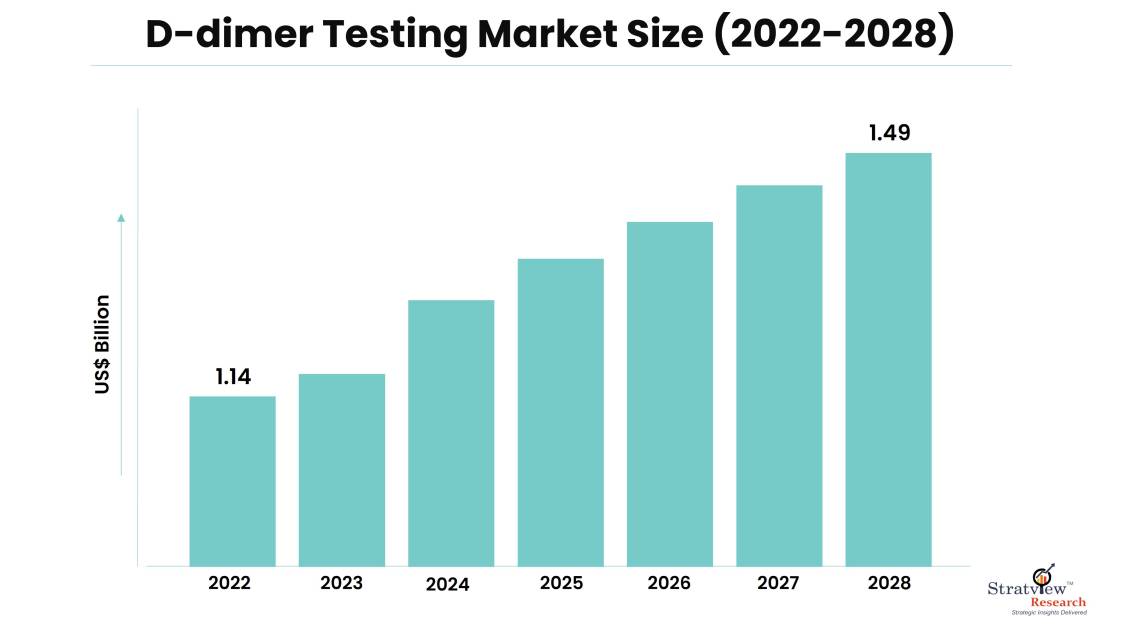
The D-dimer testing market was estimated at USD 1.14 billion in 2022 and is likely to grow at a CAGR of 4.60% during 2023-2028 to reach USD 1.49 billion in 2028.
Want to know more about the market scope? Register Here
Market Dynamics
Introduction
D-dimer testing is a blood test that is performed to measure blood ability & clotting disorder. These tests are primarily consulted by doctors during suspicion of a clotting disorder or for the diagnosis of a hemostasis system. D-Dimer is a protein fragment that has two cross-linked D fragments of protein known as fibrin. It is found in a human blood sample after the degradation of the blood clot by fibrinolysis. D-dimer testing is primarily used for the diagnosis of pulmonary embolism, deep vein thrombosis (DVT), and disseminated intravascular coagulation (DIC). These tests are conducted to identify the presence of D-dimer in the blood and locate any chronic clots in the body. These tests involve the emergence of monoclonal anti-D-dimer bodies with latex particles, which improves the molecular weight of the immunocomplexes in the test sample.
Covid-19 Impact Analysis
The outbreak of COVID-19 moderately affected the D-dimer testing industry. The healthcare industry is currently facing major challenges as it is unable to provide proper medical facilities to meet the ever-growing coronavirus patient pool. With the stringent imposition of lockdown and social distance regulations, there were limited testing and appointments for blood clots, thus affecting market growth.
Market Drivers
The D-dimer testing market is driven by a host of factors, some of which are noted below:
- A significant shift towards the demand for next-generation point-of-care D-dimer testing.
- This is mainly due to the implementation of POC solutions substantially lowering waiting time, enabling faster diagnosis, reducing the length of patient stay, and leading to improved patient experience.
- The benefits gained from these solutions in POC settings, as well as the rising incidence rate of Venous Thromboembolism (VTE) along with several pulmonary & cardiovascular diseases, which cause life-threatening issues owing to blood clot formation, is increasing the demand for D-dimer testing.

Want to have a closer look at this market report? Register Here
Key Players
The following are some of the major players operating in the D-dimer testing market are:
- Laboratory Corporation of America Holdings (US)
- Response Biomedical Corp (Canada)
- Unbound Medicine, Inc (US)
- Helena Laboratories (US)
- Siemens Healthcare Diagnostics (US)
- Trinity Biotech plc (Ireland)
- Hoffmann-La Roche Ltd (Switzerland)
- Abbott Laboratories, Inc (US)
- Eurolyser Diagnostica GmbH (Austria)
- ARUP Laboratories (US)
Note: The above list does not necessarily include all the top players in the market.
Are you the leading player in this market? We would love to include your name. Write to us at sales@stratviewresearch.com
Segments Analysis
|
Segmentations
|
List of Sub-Segments
|
Segments with High Growth Opportunity
|
|
Test Type Analysis
|
Clinical Laboratory Tests, Point-of-Care Tests
|
The clinical laboratory tests segment dominated the market for D-dimer testing in 2022 and accounted for a revenue share of 55% which is expected to remain dominant during the forecast period.
|
|
Application Type Analysis
|
Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Others
|
The Deep Vein Thrombosis (DVT) segment dominated the market and accounted for a revenue share of more than 30% in 2022 and is expected to remain the largest market during the forecast period.
|
|
Regional Analysis
|
North America, Europe, Asia-Pacific, and Rest of the World
|
North America accounted for the largest share of more than 30% in 2022 and is expected to grow at a significant CAGR during the review period.
|
By Test Type
The market has been divided into clinical laboratory tests and point-of-care tests. The clinical laboratory tests segment dominated the market for D-dimer testing in 2022 and accounted for a revenue share of 55%, which is expected to remain dominant during the forecast period.
Highly sensitive D-dimer assays are generally used for the exclusion of VTE as well as for the management of disseminated intravascular coagulation. Clinical laboratories have a higher frequency of tests and are well-equipped with medical facilities, thereby augmenting segment growth.
By Application Type
The market has been classified into deep vein thrombosis (DVT), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), & others. Under these, the Deep Vein Thrombosis (DVT) segment dominated the market and accounted for a revenue share of more than 30% in 2022 and is expected to remain the largest market during the forecast period.
DVT is a frequent medical ailment that, due to immobility, primarily affects hospitalized patients. This increases the likelihood of DVT and the risk of complications connected with it.
Patients who have had various types of surgery are at a higher risk of acquiring DVT. According to research, patients having spinal interbody fusion are at a high risk of getting lower limb DVT. DVT also necessitates a longer hospital stay and pharmaceutical therapy that can last many months, adding to the overall healthcare expenditure. As a result, early DVT management is critical, which increases the demand for D-dimer testing on a wide scale.
Regional Insights
North America accounted for the largest share of more than 30% in 2022 and is expected to grow at a significant CAGR during the review period.
The market growth is mainly propelled by well-equipped healthcare infrastructure, highly qualified medical professionals, the rising prevalence of blood clots in patients in the region, and increasing healthcare expenditure, further bolstering regional market growth.
Asia-Pacific is expected to grow at the highest CAGR during the review period on account of the growth of the major end-use industries. This growth is mainly attributed to the presence of a large patient pool in the developing economies of China and India.
Also, the presence of supportive guidelines for coagulation tests laid down by private organizations & government bodies, coupled with surging funds for healthcare facilities, further proliferates the regional market expansion.

Know the high-growth countries in this report. Register Here
Research Methodology
- This strategic assessment report from Stratview Research provides a comprehensive analysis that reflects d-dimer testing market realities and future market possibilities for the forecast period.
- The report segments and analyzes the market in the most detailed manner in order to provide a panoramic view of the market.
- The vital data/information provided in the report can play a crucial role for market participants as well as investors in the identification of the low-hanging fruits available in the market as well as to formulate growth strategies to expedite their growth process.
- This report offers high-quality insights and is the outcome of a detailed research methodology comprising extensive secondary research, rigorous primary interviews with industry stakeholders, and validation and triangulation with Stratview Research’s internal database and statistical tools.
- More than 1,000 authenticated secondary sources, such as company annual reports, fact books, press releases, journals, investor presentations, white papers, patents, and articles, have been leveraged to gather the data.
- We conducted more than 15 detailed primary interviews with market players across the value chain in all four regions and industry experts to obtain both qualitative and quantitative insights.
Report Features
This report provides market intelligence in the most comprehensive way. The report structure has been kept such that it offers maximum business value. It provides critical insights into market dynamics and will enable strategic decision-making for existing market players as well as those willing to enter the market. The following are the key features of the report:
- Market structure: Overview, industry life cycle analysis, supply chain analysis.
- Market environment analysis: Growth drivers and constraints, Porter’s five forces analysis, SWOT analysis.
- Market trend and forecast analysis.
- Market segment trend and forecast.
- Competitive landscape and dynamics: Market share, Service portfolio, New Product Launches, etc.
- COVID-19 impact and its recovery curve
- Attractive market segments and associated growth opportunities.
- Emerging trends.
- Strategic growth opportunities for the existing and new players.
- Key success factors.
Market Segmentation
This report studies the market covering a period of 12 years of trend and forecast. The report provides detailed insights into the market dynamics to enable informed business decision-making and growth strategy formulation based on the opportunities present in the market.
The d-dimer testing market is segmented into the following categories:
By Test Type
- Clinical Laboratory Tests
- Point-of-Care Tests
By Application
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism (PE)
- Disseminated Intravascular Coagulation (DIC)
- Others
By Region
- North America (Country Analysis: The USA, Canada, and Mexico)
- Europe (Country Analysis: Germany, France, The UK, Russia, and the Rest of Europe)
- Asia-Pacific (Country Analysis: China, India, Australia, South Korea, and the Rest of Asia-Pacific)
- Rest of the World (Country Analysis: Saudi Arabia, Brazil, and Others)
_62482.jpg)
Click here to learn the market segmentation details.
Report Customization Options
Stratview Research offers one of the following free customization options to our respectable clients:
Company Profiling
- Detailed profiling of additional market players (up to three players)
- SWOT analysis of key players (up to three players)
Competitive Benchmarking
- Benchmarking of key players on the following parameters: Product portfolio, geographical reach, regional presence, and strategic alliances.
Custom Research: Stratview Research offers custom research services across sectors. In case of any custom research requirement related to market assessment, competitive benchmarking, sourcing and procurement, target screening, and others, please send your inquiry to sales@stratviewresearch.com.

