Market Insights
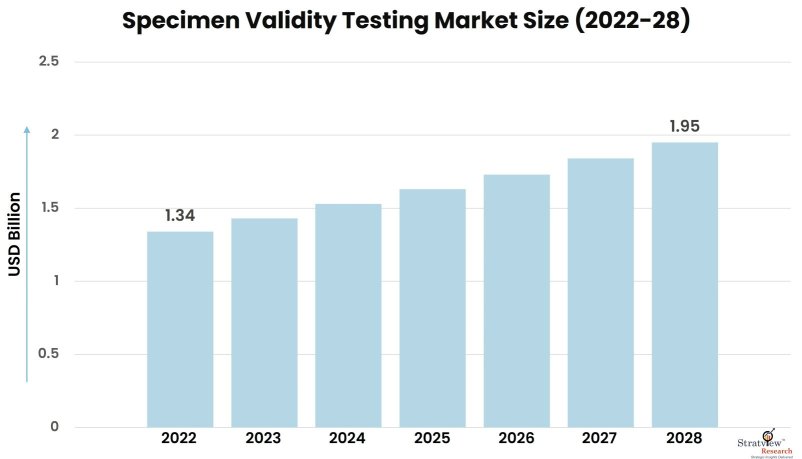
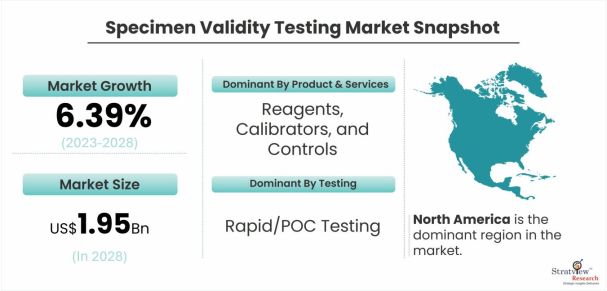
The specimen validity testing market was estimated at USD 1.34 billion in 2022 and is likely to grow at a CAGR of 6.39% during 2023-2028 to reach USD 1.95 billion in 2028.
Want to know more about the market scope? Register Here
Market Dynamics
Introduction
Specimen validity tests, also known as testing for nitrates or Test Sure, are performed on a urine drug screen specimen to detect substitution, adulteration, or dilution. A drug-testing program detects people who have used drugs. The test determines whether a urine specimen has been adulterated, diluted, or substituted.
Market Drivers
Major factors such as increasing illegal drug-related activities, stringent drug-screening regulations, and increasing government investment to combat drug abuse are expected to drive market growth during the forecast period. Furthermore, rising demand for presumptive tests, growing consumption of illicit drugs among the younger generation, and the adoption of drug screening tests at the workplace are all expected to have a significant impact on the demand for the market.
Want to have a closer look at this market report? Click Here
Key Players
Some of the key players in the market are:
- ACM Global Laboratories.
- Alfa Scientific Designs, Inc.
- American Bio Medica Corporation
- Clinical Reference Laboratory, Inc.
- GenomeWeb LLC.
- Laboratory Corporation of America
- Premier Biotech, Inc.
- Quest Diagnostics Incorporated.
- Sciteck, Inc.
- Thermo Fisher Scientific Inc.
Note: The above list does not necessarily include all the top players in the market.
Are you the leading player in this market? We would love to include your name. Write to us at [email protected]
Segments Analysis
|
Segmentations
|
List of Sub-Segments
|
Segments with High Growth Opportunity
|
|
Products & Services Type Analysis
|
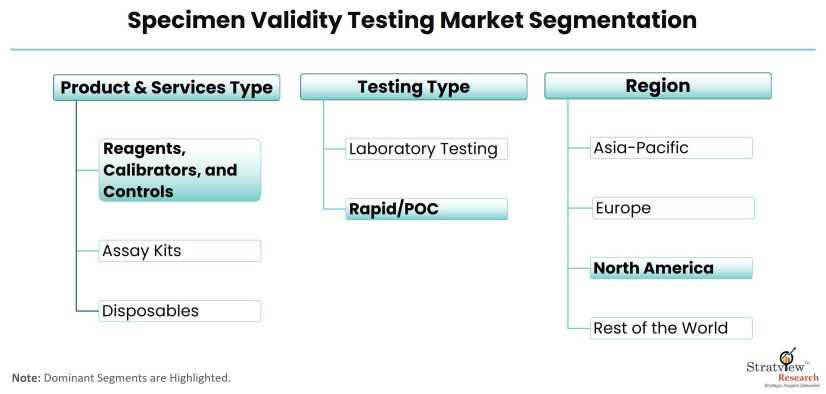
Products {Reagents, Calibrators, and Controls; Assay Kits; and Disposables} and Services
|
The reagents, calibrators, and controls sub-segment is expected to dominate the market during the forecast period.
|
|
Testing Type Analysis
|
Laboratory Testing and Rapid/POC Testing
|
Rapid/POC testing is expected to grow at a higher CAGR in the market during the forecast period.
|
|
Regional Analysis
|
North America, Europe, Asia-Pacific, and Rest of the World
|
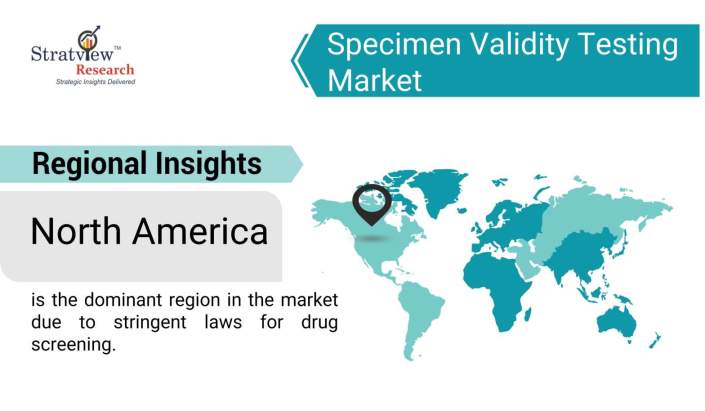
North America is the dominant region in the market.
|
By Products & Services Type
Based on the products & services type, the market is segmented as products and services. The products segment is further sub-segmented as reagents, calibrators, and controls; assay kits; and disposables. The reagents, calibrators, and controls sub-segment is expected to dominate the market during the forecast period, owing to the increasing demand for reagents and rapid screening kits in drug screening laboratories.
By Testing Type
Based on the testing type, the market is segmented as laboratory testing and rapid/POC testing. Rapid/POC testing is expected to grow at a higher CAGR in the market during the forecast period. The benefits of instant results, the ability to detect multiple drugs at once, and the recurrent and repetitive use of rapid/POC drug testing products are driving the segment's growth.
Regional Insights
In terms of regions, the market is segmented into North America, Europe, Asia-Pacific, and the Rest of the World. Among these regions, North America is the dominant region in the market due to stringent laws for drug screening, increasing government investment to control drug abuse, and an increase in the consumption of illicit drugs. Europe and Asia-Pacific are also expected to offer substantial growth opportunities during the forecast period.
Know the high-growth countries in this report. Click Here
Research Methodology
- This strategic assessment report from Stratview Research provides a comprehensive analysis that reflects specimen validity testing market realities and future market possibilities for the forecast period.
- The report segments and analyzes the market in the most detailed manner in order to provide a panoramic view of the market.
- The vital data/information provided in the report can play a crucial role for market participants as well as investors in the identification of the low-hanging fruits available in the market as well as to formulate growth strategies to expedite their growth process.
- This report offers high-quality insights and is the outcome of a detailed research methodology comprising extensive secondary research, rigorous primary interviews with industry stakeholders, and validation and triangulation with Stratview Research’s internal database and statistical tools.
- More than 1,000 authenticated secondary sources, such as company annual reports, fact books, press releases, journals, investor presentations, white papers, patents, and articles, have been leveraged to gather the data.
- We conducted more than 15 detailed primary interviews with market players across the value chain in all four regions and industry experts to obtain both qualitative and quantitative insights.
Report Features
This report provides market intelligence in the most comprehensive way. The report structure has been kept such that it offers maximum business value. It provides critical insights into market dynamics and will enable strategic decision-making for existing market players as well as those willing to enter the market. The following are the key features of the report:
- Market structure: Overview, industry life cycle analysis, supply chain analysis.
- Market environment analysis: Growth drivers and constraints, Porter’s five forces analysis, SWOT analysis.
- Market trend and forecast analysis.
- Market segment trend and forecast.
- Competitive landscape and dynamics: Market share, Service portfolio, New Product Launches, etc.
- COVID-19 impact and its recovery curve
- Attractive market segments and associated growth opportunities.
- Emerging trends.
- Strategic growth opportunities for the existing and new players.
- Key success factors.
Market Segmentation
This report studies the market covering a period of 12 years of trend and forecast. The report provides detailed insights into the market dynamics to enable informed business decision-making and growth strategy formulation based on the opportunities present in the market.
The specimen validity testing market is segmented into the following categories:
By Products & Services Type
By Testing Type
- Laboratory Testing
- Rapid/POC Testing
By End-User Type
- Workplaces
- Drug Screening Laboratories
- Criminal Justice and Law Enforcement Agencies
- Pain Management Centers
- Drug Rehabilitation Centers
- Others
By Region
- North America (Country Analysis: the USA, Canada, and Mexico)
- Europe (Country Analysis: Germany, France, the UK, Russia, Spain, and the Rest of Europe)
- Asia-Pacific (Country Analysis: China, Japan, India, South Korea, and the Rest of Asia-Pacific)
- Rest of the World (Sub-Region Analysis: Latin America, the Middle East, and Others)
Want to know the most attractive market segments? Register Here
Report Customization Options
With this detailed report, Stratview Research offers one of the following free customization options to our respectable clients:
Company Profiling
- Detailed profiling of additional market players (up to 3 players)
- SWOT analysis of key players (up to 3 players)
Market Segmentation
- Current market segmentation by any one of the products & services type by testing type.
Competitive Benchmarking
- Benchmarking of key players on the following parameters: Product portfolio, geographical reach, regional presence, and strategic alliances.
Custom Research: Stratview Research offers custom research services across sectors. In case of any custom research requirement related to market assessment, competitive benchmarking, sourcing and procurement, target screening, and others, please send your inquiry to [email protected].

